New Stem-Cell Approval Opens Way for Promising Research
By wchung | 07 Sep, 2025
Scientists can start using taxpayer dollars to do research with 13 batches of embryonic stem cells and the government says dozens more cell lines should be available soon, opening a new era for the potentially life-saving field.
President Barack Obama lifted eight years of restrictions on these master cells last spring. But $21 million-and-counting in new projects were on hold until the National Institutes of Health determined which of hundreds of existing stem cell lines were ethically appropriate to use.
“This is the first down payment,” Dr. Francis Collins, NIH’s director, said Wednesday as he opened a master registry. “People are champing at the bit for the opportunity to get started.”
Thirteen stem cell lines — created by Children’s Hospital Boston and Rockefeller University — are first on that list. Another 96 embryonic stem cell lines are undergoing NIH review, and 20 or more could get a decision by Friday, Collins said.
And researchers have notified the NIH that they may apply for approval of another 250 stem cell lines.
“The field has been waiting with bated breath for this announcement,” said Dr. George Daley of Children’s Hospital Boston, whose lab created 11 of the newly approved lines. He has about 100 vials of cells from each batch already banked and ready to ship to researchers around the country.
The numbers mark a big change from the Bush administration, which had limited taxpayer-funded research to about 21 stem cell lines, those already in existence as of August 2001. Scientists say newer batches were created in ways that made them far better candidates for successful research. Indeed, only one of the Bush-era stem cell lines is among the 96 now under consideration.
Wednesday’s announcement means that researchers who were awarded $21 million in stem cell research grants earlier this year can start using the approved lines immediately, projects that include work to one day repair damaged heart tissue and grow new brain cells. Millions more in stem cell money is due out later this winter, funds from the economic stimulus package.
Embryonic stem cells can morph into any cell of the body, and scientists hope to harness them so they can create replacement tissue to treat, possibly even cure, a variety of diseases, from diabetes to Parkinson’s to spinal cord injury.
Culling those cells destroys a days-old embryo, something many strongly oppose on moral grounds. But once created, the cells can propagate indefinitely in lab dishes.
Federal law forbids using taxpayer money to create or destroy an embryo. All the stem cell lines involved in Wednesday’s announcement were created from fertility clinic leftovers — embryos that otherwise would have been thrown away — using private money. NIH is reviewing the rest to see if they also meet ethics requirements for use in taxpayer-funded health research. Among the requirements: That the woman or couple who donated the original embryo did so voluntarily and were told of other options, such as donating to another infertile woman.
Why do scientists need so many choices? It’s not just to supply the demand of a growing field. There’s a lot of variability from batch to batch in how the stem cells perform, Daley said. Some are better at turning into blood-producing cells than muscle-producing ones, for instance.
It has to do with the genetics of the original embryo, and probably also with the recipe used to create and nurture the stem cells — an environment that can trigger genes to switch on and off at different times, explained Daley, who has government funding to study those important differences.
12/2/2009 5:44 PM LAURAN NEERGAARD, AP Medical Writer WASHINGTON
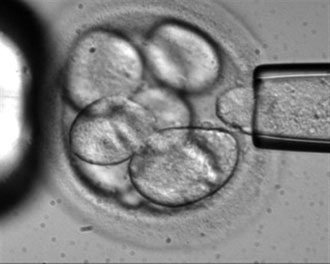
In this file photo originally made available by Advanced Cell Technology in 2006, a single cell is removed from a human embryo to be used in generating embryonic stem cells for scientific research. (AP Photo/Advanced Cell Technology)
Asian American Success Stories
- The 130 Most Inspiring Asian Americans of All Time
- 12 Most Brilliant Asian Americans
- Greatest Asian American War Heroes
- Asian American Digital Pioneers
- New Asian American Imagemakers
- Asian American Innovators
- The 20 Most Inspiring Asian Sports Stars
- 5 Most Daring Asian Americans
- Surprising Superstars
- TV’s Hottest Asians
- 100 Greatest Asian American Entrepreneurs
- Asian American Wonder Women
- Greatest Asian American Rags-to-Riches Stories
- Notable Asian American Professionals

